Original Article
Year: 2023 |Volume: 4 | Issue: 04 |Pages: 60-68
Evaluation of the pharmacokinetic aspect of Brahmi Ghrita by oral and nasal route.
About Author
Correspondence Address:
Dr. Chetan Gulhane Associate professor, professor Department of panchakarma, R A Podar Ayurveda Medical College Mumbai. Email. Drchetanayu@gmail.com
Date of Acceptance: 2023-04-15
Date of Publication:2023-05-24
Article-ID:IJIM_266_10_23 http://ijim.co.in
Source of Support: NiL
Conflict of Interest: NIL
How To Cite This Article: Gulhane CM, Thakar AB, Naria M. Evaluation of the pharmacokinetic aspect of Brahmi Ghrita by oral and nasal route. Int J Ind Med 2023;4(4):60-68
Abstract
Context: Nasya Karma is the main treatment for all the supra-clavicular diseases, i.e. disease of head, neck, mouth, eye, ear and nose. Administration of drugs by the route of nasal cavity is termed as Nasya Karma Nasal route of drug administration provides systemic absorption of drug and its fast action in low doses. But it is not clear till date the active principles of Ayurvedic formulation used in Nasya get absorbed in systemic circulation. Aim: To study comparative pharmacokinetic aspect of Brahmi Ghrita in experimental animals after nasal and oral administration and to explore the mode of action of Nasya. Materials and Methods: For pharmacokinetics study of Brahmi Ghrita same dose was use for nasal and oral administration in wistar strain albino rats. Bacoside A3 was used as marker compound and it's absorption in systemic circulation was assessed using HPTLC. Results: Bacoside A3 present in Brahmi Ghrita after nasal and oral administration was found to get absorbed in to systemic circulation. Conclusion: The intranasal administration of Brahmi Ghrita is an effective way for systemic availability of drugs and has extended drug absorption as compared to oral routes for same dose.
Keywords: Brahmi Ghrita, Nasya, Bacoside A3
Introduction
Administration of drugs by the route of nasal cavity is termed as Nasya Karma1; it is the main treatment for all the supra- clavicular diseases, i.e. disease of head, neck, mouth, eye, ear and nose. Acharya Charaka included Brahmi in the list of Shirovirechana Dravya (medicine used for nasal administration).2 Brahmi (Bacopa moniera Linn.) is a Medhya (cognitive) drug described in Ayurvedic classics.3 Bacopa moniera (Brahmi) is a good Nootropic4,5 (smart drugs, memory enhancers, and cognitive enhancers as well as intelligence enhancers). Its traditional use is as an important Ayurvedic traditional herbal medicine. It is used as a nerve and brain tonic to improve memory, learning and concentration. In addition, it repairs nerve damage, as well as stroke and brain injury. Some experimental studies show Brahmi Improves Learning and Memory in Mice.6 Bacosides which are active ingredients of Brahmi repair damaged neurons by boosting kinase, which is the protein used to synthesize new neurons. It improves the synaptic activity and thus memory can be restored.7
Many experimental studies has been carried out in modern medicine which show that nasal delivery has been explored as an alternative administration route to target drugs directly to the brain.8 But till date no experimental study is carried out using Ayurvedic preparation like Brahmi Ghrita Nasya and also it is not clear that when Nasya is given the active principles in the formulation gain entry in to the systemic circulation or not. The present study was planned to acquire some preliminary data with regards to the absorption of phytochemical constituents of the formulations when administered in the form of Nasya. The aim of this study was to explore the pharmacokinetic of Brahmi Ghrita Nasya using Bacoside A3 in experimental animals.
Aim and objective: To study comparative pharmacokinetic aspect of Brahmi Ghrita in experimental animals after nasal and oral administration.
MATERIAL & METHODS:
Test formulation
The fresh Bhrahmi (Bacopa moniera Linn.) was collected from Jamnagar and authenticated by pharmacognocy department. Brahmi Ghrita9 was prepared in the pharmacy of ITRA, Gujarat Ayurved University, Jamnagar.
Animals: Healthy Wistar strain albino rats weighing between 280 ± 10g were of either sex were used for the experiments. The experimental protocols were approved by Institutional Animal Ethics Committee (IAEC/10/2012/04) in accordance with the guideline formulated by CPCSE, India.
Husbandry conditions: Animals were kept in polypropylene cage with top stainless steel grill. Peddy husk was used as bedding material for animals. The animals were exposed to 12 hours light and 12 hours dark cycle with the relative humidity of 50 to 70% and the ambient temperature was 22 ± 03ºC. All animals were kept on same environmental conditions. The selected animals were fasted over night before experiment.
Diet: Amrut brand rat pellet feed supplied by Pranav Agro Ltd. was provided throughout the study period. Drinking water was given ad libitum in polypropylene bottles with stainless steel sipper tube. The animals were fasted overnight before experimentation.
Acclimatization period: All the selected animals were kept under acclimatization for seven days before experimentation.
Numbering & Identification: The animals were marked with saturated picric acid for proper identification.
Dose fixation: Dose of the drug was calculated by extrapolating the human therapeutic dose to rat and mice on the basis of body surface area ratio (conversion factor 0.018 for rat) by referring to the table of Paget and Barnes (1964)10
Brahmi Ghrita and Plain Ghrita : The adult dose of Ghrita is 16ml11,12 for nasal and oral administration.
Rat dose = Therapeutic human dose × conversion factor for rat of 200 g
= 16 ml × 0.018 = 0.288 ml for 200 g rat = 1.44 ml/kg body weight of rat
Route of drug administration: The Ghrita administered according to the body weight of the animals by oral route with the help of oral feeding canula and nasal administration by micropipette. The test drug and vehicle were administered between 08:30 am to 09:30 am.
Instruments used: Weighing scale, micro-titre plates, serological water bath, digital Plethysmograph, burette, needle, syringe, surgical instruments, centrifuge machine, refrigerator, water bath etc.
Experimental protocol: The selected animals were grouped randomly irrespective of sex into two groups each consist of three animals. Group I received Brahmi Ghrita through Nasya route (1.44 ml/kg) and Group II received Brahmi Ghrita through oral route (1.44 ml/kg). In the morning initial blood sample was collected through retro-orbital puncture under light ether anaesthesia. Afterwards the Brahmi Ghrita was administered through oral and nasal route to respective groups. Blood samples were again taken at regular interval of post-dose (after drug administration), 30 mins, 60 mins, 120 mins and 180 mins for estimation of biomarker in the serum through HPTLC method. From the blood samples, serum was separated by centrifuging at 3000 rpm for 10 minutes. The serum samples were subjected to HPTLC analysis to know absorption of Brahmi Ghrita through nasal and oral route in to systemic circulation by using Bacoside A3 as marker compounds.
Chromatographic conditions:
- Application mode : CAMAG Linomat V Hamilton Syringe
- Development chamber : CAMAG Twin trough chamber (20 x 10 cm2)
- Plates : Precoated silica gel GF254 plates
- Chamber saturation : 30 min
- Development distance : 8 cm
- Development time : 30 min
- Scanner : CAMAG TLC Scanner III
- Scanning mode : Linear at 254 nm and 366 nm
- Detection : Deuterium lamp, Mercury lamp
- Photo documentation : CAMAG reprostar
- Data system : CATS software (Ver. 3.17)
- Drying device : Oven
- U.V. Spectrum : 200 nm to 700 nm
Steps involved in HPTLC:
- Selection of chromatographic layer.
- Sample and standard preparation.
- Layer pre-washing, Layer pre-conditioning.
- Application of sample and standard.
- Chromatographic development.
- Detection of spots.
- Scanning.
- Documentation of chromatic plate.
Serum sample preparation:
- 1ml serum was adjusted to pH 2.5 with 300μl of 1 mol potassium dihydrogen phosphate solution & 30μl phosphoric acid.
- 5ml of acetonitrile was added and vertex mixed for 1min. It forms a precipitate.
- It was centrifuged at 3500 r.p.m. for 15min at 8-100C.
- Supernatant liquid is evaporated to dryness at 370C.
- Residue reconstituted in 1 ml methanol.
- The solution was filtered through Whatman filter paper No. 41 in a dry stoppered tube & stored at 0-40C for further analysis. This solution was used for HPTLC analysis.
Standard preparation: Reference standard of Bacoside A3 were provided by M/s Natural remedies vt.Ltd. Bangalore India. Standard was prepared in alcohol (Std.S s 1mg/ml)
Sample for HPTLC
For HPTLC study following samples were titled as track 1-13
Track-1 : Processed serum sample of before nasal administration of Brahmi Ghrita
Track-2 : Processed serum sample of before oral administration of Brahmi Ghrita
Track-3: standard sample of Bacoside A3 2.5 µg (Std.S s 1mg/ml)
Track-4: standard sample of Bacoside A3 5.0 µg
Track-5 : standard sample of Bacoside A3 7.5 µg
Track-6: Processed serum sample of after 30 min of nasal administration of Brahmi Ghrita
Track-7: Processed serum sample of after 30min of oral administration of Brahmi Ghrita
Track-8: Processed serum sample of after 60 min of nasal administration of Brahmi Ghrita
Track-9: Processed serum sample of after 60 min of oral administration of Brahmi Ghrita
Track-10: Processed serum sample of after 120 min of nasal administration of Brahmi Ghrita
Track-11: Processed serum sample of after 120 min of oral administration of Brahmi Ghrita
Track-12: Processed serum sample of after 180 min of nasal administration of Brahmi Ghrita
Track-13: Processed serum sample of after 180 min of oral administration of Brahmi Ghrita
Selection of mobile phase
In liquid chromatography, the solute retention is governed by the solute distribution factor, which reflects the different interactions of the solute - stationary phase, the solute - mobile phase and the mobile phase – stationary phase. For a given stationary phase, the retention of the given solute depends directly upon the mobile phase, the nature and the composition of which has to be judiciously selected in order to get appropriate and required solute retention. The mobile phase has to be adapted in terms of elution strength (solute retention) and solvent selectivity (solute separation). Solvent polarity is the key word in chromatographic separations since a polar mobile phase will give rise to low solute retention in normal phase and high solute retention in reversed phase LC.
Mobile phase for present study:
Mobile phase: Dichloromethane (85) : Methanol (15) : Acetic acid ( 5 V/V)
Procedure and Data Analysis:
The Chromatographic System was set up as above. Separately injected equal volumes (about 20µl) of the Standard preparation and the serum sample preparation into the chromatograph, the chromatograph for qualitative percentage of Bacoside A3 were recorded and any peaks from the Mobile phase preparation were discarded. Quantitative determination was carried out with previously used external standard method13 using Microsoft excel sheet. Maximum serum concentration was determined by visual inspection of the Data.
tables
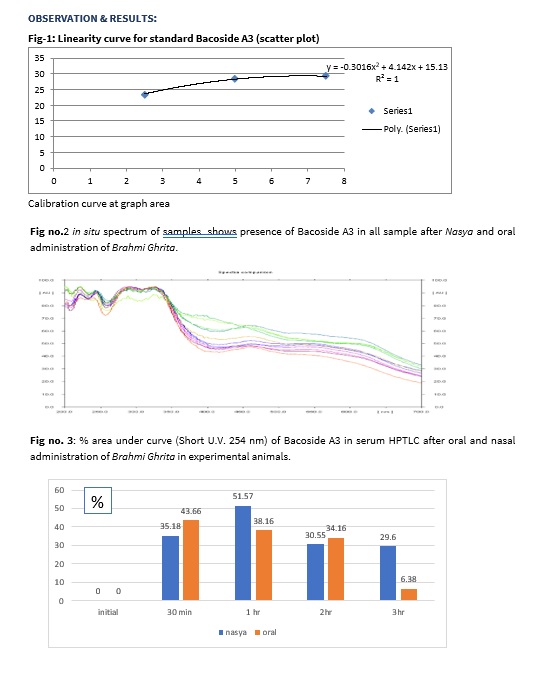
In Short U.V. (254 nm) maximum concentration of Bacoside A3 at Rf value 0.95 and 0.94 was observed in standard sample of Bacoside A3. Serum samples of collected from both groups also showed the same Rf values for maximum concentration of Bacoside A3. From the above shown graph, it is clear the Bacoside A3 were present in the plasma of both group samples after oral and nasal administration of Brahmi Ghrita collected at various time intervals. Brahmi Ghrita through oral route showed that maximum concentration of Bacoside A3 was appeared in serum sample after 30 mins (43.66%) and thereafter the concentration was gradually decrease after 1 hr (38.16%), 2 hrs (34.16%) and 3 hrs (6.38%). Brahmi Ghrita through nasal route showed that maximum concentration of Bacoside A3 was appeared in serum sample after 1 hr and thereafter the concentration was gradually decrease after 2 hrs (30.55%) and 3 hrs (29.60%).
Discussion
Intranasal route has promising approaches for delivery of drugs to the brain. The delivery of drugs to the CNS from the nasal route may occur via olfactory neuroepithelium. The intranasal administration of drugs is an effective way for systemic availability of drugs as compared to oral and intravascular routes. Actually, it seems to present fast and extended drug absorption.14 This experimental study is carried out for to study the pharmacokinetic Brahmi Ghrita after nasal and oral administration with same dose. Bacoside A3 was present in the serum sample of rats after 30 mins of drug administration through oral and nasal routes. This indicate that drug have fast absorption though both routes in experimental animals. Brahmi Ghrita through oral route showed that maximum concentration of Bacoside A3 in serum was observed after 30 min and thereafter gradually decrease after 1 hr, 2 hrs. and 3 hrs of drug administration. After 3 hrs the concentration of Bacoside A3 was almost decrease which indicate the diminution of active component of Brahmi Ghrita through oral route after 3 hrs. Brahmi Ghrita through nasal route showed that concentration of Bacoside A3 in serum was increased from 30 mins to 1 hr. at maximum level and thereafter gradually decreases after 2 hrs and 3 hrs of drug administration. After 3 hrs, the concentration of Bacoside A3 was still 26.9% in serum which indicates that drug through nasal route may have extended efficacy compare to oral route.
Lipophilic drugs are well absorbed from the nasal cavity, exhibiting pharmacokinetic profiles similar to those obtained after intravenous administration. These drugs are absorbed quickly and efficiently across the nasal membrane via trans cellular mechanisms. This observation is true for lipophilic compounds having molecular weight lower than 1 kDa. On the other hand, the rate and degree of nasal absorption of polar drugs is low and highly dependent of the molecular weight. Drug absorption is expected to be diminished with decrease lipophilicity because the nasal membrane is lipophilic. Whenever lipophilicity is too high, the drug permeation through the wall may be reduced because drug does not dissolve easily in the aqueous environment of nasal cavity.15 Oil and Ghee preparations are more viscous in nation it is proved that formulation with higher viscosity has a better contact time thus increases the absorption. At the same time, high viscosity enhanced the permeability of drugs.16
Clinically also nasal administration of Brahmi Ghrita effective against oral same dose. Relatively low doses are effective when administered through nasal route with less systemic toxic effects. It is proved that the intranasal administration of drugs is an effective way for systemic availability of drugs as compared to oral and intravascular routes. Actually, it seems to present fast and extended drug absorption, and it has been supported by many studies planned to compare intranasal drug delivery against oral and parenteral administration.17
Conclusion
From the present study, it is concluded that Brahmi Ghrita is absorbed in systemic circulation from oral and nasal route in rats. The intranasal administration of drugs is an effective way for systemic availability of drugs as compared to oral and intravascular routes for same dose. Actually, it seems to present fast and extended drug absorption. The intranasal route is an accessible alternative route for Brahmi Ghrita drug administration. This route provides future potential for several drugs through the development of safe and efficacious formulations for simple, painless and long?term therapy. From this route drugs can be directly target to the brain in order to attain a good therapeutic effect in CNS with reduced systemic side effects.
References
- Vaghbhata, Ashtanga Samgraha "Nasya Vidhi Suthrasthana 29/2". In Sharma, Shivprasad. Ashtanaga Samgraha Indu virachita Sasilekha vyakhyaya samanvita. Varanasi, India: Chowkhamba Sanskrit Series Office. (2012). p. 223.
- Agnivesha, Charaka, Dridhabala, Charaka Samhita, Viman Sthana, Rogabhishagjitiya Viman Adhyaya,8/151, edited by Aacharya Vidyadhar Shukla, first edition, Chaukhamba Sanskrit Pratishthana Varanasi, 2002;666
- Acharya Priyavata Sharma, Dravyaguna Vigyan, vol. II, Chaukhambha Bharati Academy, Varanasi 2009;8
- S. Roodenrys, D. Booth, S. Bulzomi, A. Phipps, C. Micallef, J. Smoker (2002). "Chronic effects of Brahmi (Bacopa monnieri) on human memory". Neuropsychopharmacology (Wollongong) 27 (2): 279.
- Stough C, Downey LA, Lloyd J et al. (2008). "Examining the nootropic effects of a special extract of Bacopa Monniera on human cognitive functioning: 90 day double-blind placebo-controlled randomized trial." Phytother Res. 22:1629-1634
- Hanumanthachar Joshi, Milind Parle, Brahmi rasayana Improves Learning and Memory in Mice, Evid Based Complement Alternat Med. 2006 March; 3(1): 79–85. Published online 2006 January 16.
- Zac Bobrov, Herbs and nutrients are good for the brain, Posted: 28 Oct, 2008 http://thephj.com/features/article/herbs_and_nutrients_are_good_for_the_brain.
- Michael Ikechukwu Ugwoke, et al. The biopharmaceutical aspects of nasal mucoadhesive drug delivery, Journal of Pharmacy and Pharmacology, Volume 53, Issue 1, pages 3–22, January 2001
- Sharangadhara, ‘Sharangadhara Samhita’, with ‘Krishana’ Hindi commentary, by Sri Radhakrishana Parashara,,fourth edition, Sri Baidyanatha Ayurveda Bhavana, Great Naga Road, Nagpur-9,(India),1994, Madhyama Khanda, Sneha kalpana, 9/1,pg.321
- Paget, G.E., Barnes, J.M. Evaluation of drug activities.InPharmacometrics. Edited by Laurence D.R. Bacharach AL. Academic Press, London 1964;1:50.
- Sushruta , Dalhana , Sushrut Samhita, Dhoom Nasya Kavalgraha
- ChikitstaAdhyaya,40/23,edited by P. G. Aathavale, 2nd edition, Godavari publishers and book promoters, Nagpur,2008;279
- Ganesh B., Jaydevan, et al. “standardization of the dose of Nasya karma”. Kottakkal; 2011
- United States Pharmacopoeia, 24 NF (19), The United States Pharmacopoeia Convention Inc. USA, 2000, 17, 403, 546
- Shao Z, Park GB, Krishnamoorthy R, Mitra AK. The physicochemical properties, plasma enzymatic hydrolysis, and nasal absorption of acyclovir and its 2’ ester prodrugs. Pharm Res, 1994; 11: 237?242.
- Sharma PK, Chaudhari P, Kolsure P, Ajab A, Varia N. Recent trends in nasal drug delivery system ? an verview. 2006; 5: vol 4
- Zaki NM, Awad GA, Mortada ND, Abd ElHady SS. Rapid onset intranasal delivery of metoclopramide hydrochloride. Part I. Influence of formulation variables on drug absorption in anesthetized rats. Int J Pharm, 2006; 327: 89 96.
- Stoke DG, Reber KR, Waltzman LS, Erns C, Hamilton D, Gawareck D, Mermelstein F, McNicol E, Wright C, Carr DB. Analgesic efficacy and safety of morphine chitosan nasal solution in patients with moderate to severe pain following orthopedic surgery. Pain Med, 2008; 9: 312
"

