Original Article
Year: 2025 |Volume: 6 | Issue: 10 |Pages: 77-81
In-Vitro Antimicrobial Efficacy of Marichyadi Taila
About Author
Correspondence Address:
Dr. Amit Kapila Associate Professor, Rasa Shastra Evam Bhaishajya Kalpana, Desh Bhagat Ayurvedic College & Hospital, Punjab, India Email: kapilaamit2@gmail.com
Date of Acceptance: 2025-10-15
Date of Publication:2025-11-20
Article-ID:IJIM_463_11_25 http://ijim.co.in
Source of Support: Nill
Conflict of Interest: Nill
How To Cite This Article: Kapila A., In-Vitro Antimicrobial Efficacy of Marichyadi Taila. Int J Ind Med 2025;6(10):77-81 DOI: http://doi.org/10.55552/IJIM. 2025.61016
Abstract
Marichyadi Taila is a polyherbal Ayurvedic oil formulation traditionally prescribed for various dermatological disorders (Kustha roga), many of which have microbial etiology. The rising concern over antimicrobial resistance (AMR) necessitates the scientific validation of traditional medicines. This study aimed to evaluate the in-vitro antibacterial and antifungal efficacy of three different batches (3698, 3718, and 3736) of Marichyadi Taila against common pathogenic microorganisms: Escherichia coli (Gram-negative bacteria), Staphylococcus aureus (Gram-positive bacteria), and Candida albicans (fungus). The antimicrobial activity was assessed using the Cup Plate Agar Diffusion method, comparing the zones of inhibition (ZOI) against standard control drugs (Gentamycin for bacteria and Fluconazole for fungi). The results indicated that Marichyadi Taila possesses moderate, dose-dependent antimicrobial activity against all tested strains. The maximum inhibitory efficiency was observed against C. albicans, with Batch No. 3718 showing the highest antifungal efficiency at 62.50% compared to Fluconazole. Against bacterial strains, the oil demonstrated greater efficacy against the Gram-positive S. aureus (up to 38.46% efficiency) than against the Gram-negative E. coli (up to 33.33% efficiency). Although its potency was significantly lower than the synthetic standard drugs, the consistent activity across all batches confirms the inherent broad-spectrum antimicrobial properties of Marichyadi Taila, supporting its traditional use in the management of infectious skin conditions.
Keywords: Marichyadi Taila, Escherichia coli (Gram-negative bacteria), Staphylococcus aureus (Gram-positive bacteria), and Candida albicans (fungus).
Introduction
Traditional medicine systems, such as Ayurveda, have been utilized for millennia to treat a wide array of human ailments, often relying on complex polyherbal formulations. In the context of dermatological care, Ayurvedic texts describe numerous oil-based preparations (Tailas) designed for topical application to manage chronic and infectious skin conditions classified under Kustha roga. Among these formulations, Marichyadi Taila holds a prominent position, particularly indicated for infections like dadru (ringworm) and pama (scabies), conditions intrinsically linked to bacterial and fungal pathogens.1
The global health community faces a critical challenge due to the proliferation of drug-resistant microorganisms. The efficacy of conventional antibiotics is rapidly diminishing, driving the urgent need to explore new antimicrobial agents from natural sources. Herbal preparations, with their complex synergy of multiple bioactive compounds, offer a promising alternative or complementary therapeutic approach.
Marichyadi Taila is a classical preparation comprised of a base oil (Mustard oil) processed with a decoction and a kalka (paste) of several highly potent plant and mineral ingredients. The formulation includes established antimicrobial agents like Haridra (Curcuma longa), Chandana (Santalum album), Devadaru (Cedrus deodara), and Maricha (Piper nigrum), alongside potent detoxifying minerals such as Haritala (Yellow Arsenic) and Manahshila (Red Arsenic), used strictly for external applications in minute, purified forms as per classical guidelines. The vehicle, Murchit Katu tail (Mustard oil), itself possesses known antifungal and antipathogenic properties, suggesting a cumulative synergistic effect.2
The present study systematically investigates the in-vitro antimicrobial potential of Marichyadi Taila to provide scientific evidence for its traditional therapeutic claims. The primary focus is on quantifying its inhibitory effects against common skin-related pathogens and establishing a comparative baseline against modern pharmaceutical standards.3
2. Aims and Objectives
-
To determine the antibacterial activity of the oil against the Gram-negative bacteria Escherichia coli and the Gram-positive bacteria Staphylococcus aureus.
-
To determine the antifungal activity of the oil against the yeast Candida albicans.
-
To compare the efficacy of the test samples with standard synthetic antimicrobial controls (Gentamycin and Fluconazole).
3. Materials and Methods
3.1. Materials Required for Marichyadi Taila Preparation4
The Marichyadi Taila was prepared using the following 18 ingredients, in accordance with the classical Ayurvedic pharmacopoeia, which ensures all physiochemical parameters comply with safety standards.
|
Sl. No. |
Name of Drugs |
Scientific Name |
Part Used |
Quantity |
Properties |
|
1 |
Murchit Katu tail/Sarshapataila5 |
Mustard oil |
Oil |
768 ml |
Antifungal & Antipathogenic |
|
2 |
Visha (shuddha Vatsnabha) |
Aconitum ferox Wall ex Ser. |
Root |
48 g |
Reduces pain and inflammation |
|
3 |
Gomutra |
Cow’s Urine |
- |
3 L |
Antimicrobial, antifungal, and anticancer |
|
4 |
Maricha Churana |
Piper nigrum Linn. |
Fruit |
24 g |
Anti-inflammatory |
|
5 |
Haritala |
Yellow arsenic |
- |
24 g |
Therapeutic uses in Kustha, Dadru |
|
6 |
Manahshila |
Red arsenic |
- |
24 g |
Uses in skin diseases |
|
7 |
Musta |
Cyperus rotundus Linn. |
Rhizome (Rz.) |
24 g |
Useful in pruritus, scabies |
|
8 |
Arka payas |
Calotropis procera (Ait) R.Br. |
Latex (L.) |
24 g |
Protection against inflammation & Analgesic |
|
9 |
Karavira |
Nerium indicum Mill. |
Root (Rt.) |
24 g |
Used in very chronic skin diseases |
|
10 |
Jatamansi |
Nardostachys jatamansi D.C. |
Rt. Rz. |
24 g |
Antifungal |
|
11 |
Trivrutta |
Operculina turpethum (Linn.) |
Root (Rt.) |
24 g |
Anti-inflammatory activity |
|
12 |
Gomaya rasa |
Cow’s faeces |
- |
24 g |
Anti-microbial |
|
13 |
Vishala (indervaruni) |
Citrullus colocynthis Schrad. |
Root (Rt.) |
24 g |
Anti-inflammatory and anti-septic |
|
14 |
Kushtha |
Saussurea lappa C.B. Clarke |
Root (Rt.) |
24 g |
Antiviral |
|
15 |
Haridra |
Curcuma longa Linn. |
Rhizome (Rz.) |
24 g |
Antifungal |
|
16 |
Daru haridra |
Berberies aristata DC |
Stem (St.) |
24 g |
Anti-microbial |
|
17 |
Devadaru |
Cedrus deodara (Roxb.) Loud |
Heartwood (Hd.wd.) |
24 g |
Antifungal & Antibacterial |
|
18 |
Chandana |
Santalum alba Linn. |
Heartwood (Hd.wd.) |
24 g |
Antibacterial & Antifungal activity |
3.2. In-Vitro Antimicrobial Study Methodology6,7
3.2.1. Culture Media and Microorganisms8
The study utilized standard microbial strains:
-
Bacteria: Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). Cultured on Soybean Casein Digest Agar Media.
-
Fungus: Candida albicans (Yeast). Cultured on Potato Dextrose Agar Media.
3.2.2. Sample and Standard Preparation
-
Marichyadi Taila Sample: 10 mg of the oil sample was accurately weighed and dissolved in 10 ml of Dimethyl Sulfoxide (DMSO) to prepare a stock concentration of 1 mg/ml. DMSO was chosen as a solvent due to its low microbial inhibitory effect.
-
Standard Preparation: Gentamycin and Ciprofloxacin (antibacterial) and Fluconazole (antifungal) standard solutions were prepared at a concentration of 1 mg/ml to serve as positive controls.9
3.2.3. Subculturing and Inoculum Preparation
Agar slants were prepared by sterilizing melted agar media in test tubes and allowing them to solidify in an inclined position. Stock cultures of S. aureus and A. niger (used here as a general method description for fungi, though C. albicans was tested) were subcultured onto these slants under aseptic conditions and incubated for 24 hours at 30-37°C. The working inoculum for the assay was then prepared by transferring a small amount of the microbial culture from the agar slant into 5-10 ml of sterile Soybean Broth, ensuring proper suspension and incubation for microbial growth.
3.2.4. Cup Plate Method (Agar Diffusion Method)10
The antimicrobial activity was determined using the Cup Plate Diffusion method.
-
Plate Preparation: Sterilized media (Soybean Casein Digest Agar for bacteria, Potato Dextrose Agar for fungi) were poured into Petri plates and allowed to solidify.
-
Well Creation: Using a sterile cavity borer, wells (or 'cups') were meticulously created in the solidified, inoculated media.
-
Sample Introduction: The prepared 1 mg/ml stock solutions of the test samples (Marichyadi Taila), standard controls (Gentamycin/Fluconazole), and the solvent blank (DMSO) were introduced into the respective wells using a micropipette, ensuring equal volumes in each well.
-
Incubation: The plates were incubated at the appropriate temperature for 18-24 hours, allowing for microbial growth and diffusion of the test substances.
-
Measurement: Post-incubation, the plates were examined for the formation of clear, circular zones of inhibition (ZOI) around the wells. The diameter of the ZOI was measured using an antibiotic zone reader and recorded in millimeters (mm).
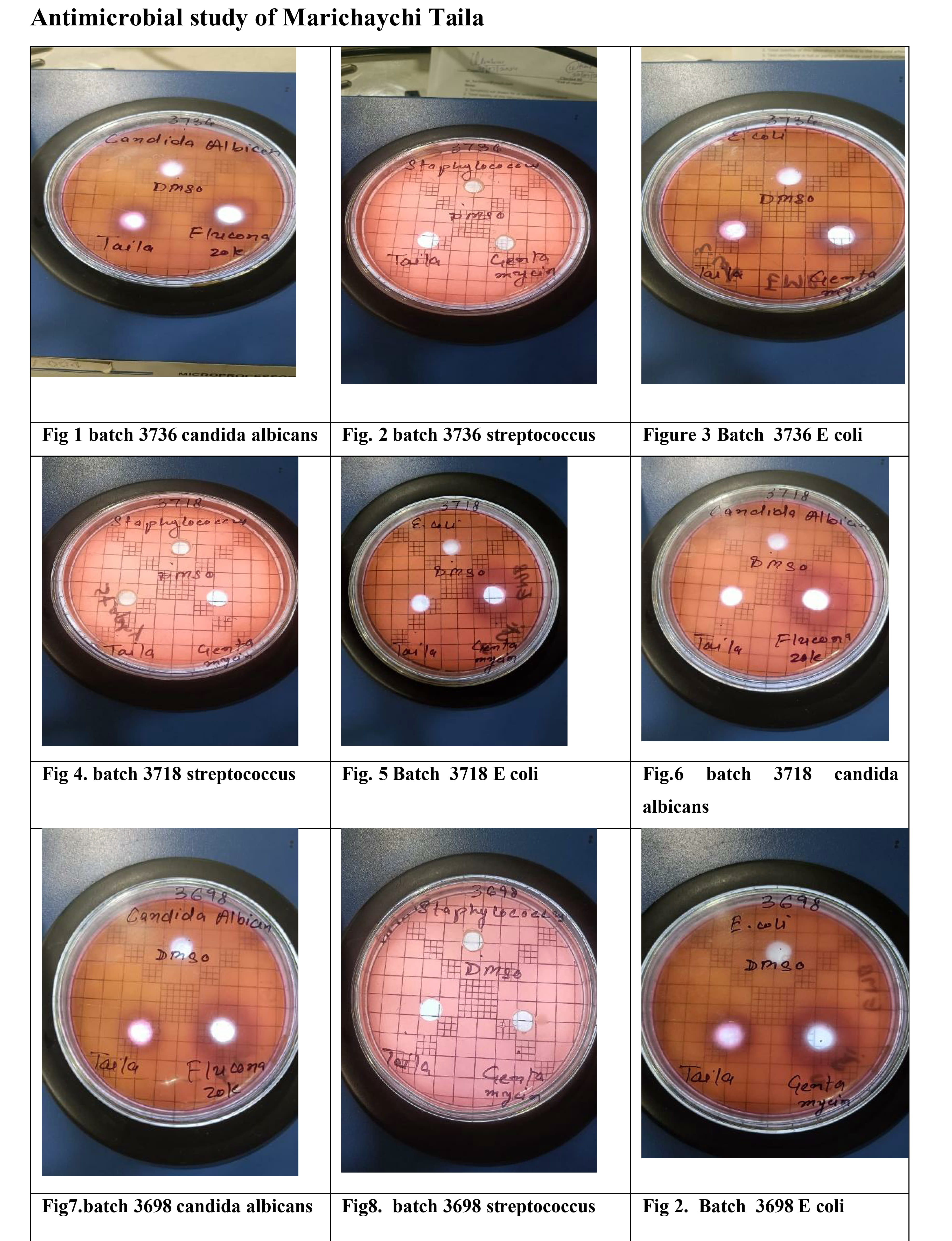
4. Observations and Results
The study evaluated the antimicrobial efficacy of three different batches of Marichyadi Taila (3698, 3718, 3736) against three microorganisms. The results are summarized below, comparing the ZOI of the test samples against the standard controls.
4.1. Antibacterial Activity: Escherichia coli
The test formulation showed mild to moderate inhibitory activity against E. coli. The solvent control, DMSO, showed a minimal, negligible inhibition zone (2-3 mm).
Analysis: Batch No. 3698 exhibited the highest ZOI (8 mm) against E. coli, corresponding to 33.33% of the Gentamycin control's efficacy. Batch No. 3718 displayed the lowest activity (6 mm). Gentamycin consistently showed superior antibacterial efficacy (23-24 mm ZOI).
4.2. Antibacterial Activity: Staphylococcus aureus
The oil formulation demonstrated slightly better inhibitory activity against the Gram-positive S. aureus compared to E. coli.
Analysis: Batches 3698 and 3718 showed identical strong inhibition (11 mm) against S. aureus, representing 37.93% of the Gentamycin efficacy. Batch 3736 showed a 10 mm ZOI, which, when compared against its respective Gentamycin control (26 mm), yielded the highest percentage inhibition efficiency (38.46%). This consistent performance suggests that the formulation is generally more effective against Gram-positive than Gram-negative bacteria.
4.3. Antifungal Activity: Candida albicans
The most notable antimicrobial activity of Marichyadi Taila was observed against the fungal pathogen C. albicans. The DMSO control showed 5-6 mm inhibition, which is still negligible compared to the test samples.
Analysis: Marichyadi Taila demonstrated strong antifungal properties. Batch No. 3718 exhibited the maximum ZOI (15 mm), achieving an impressive 62.50% of the Fluconazole control’s potency. Batch No. 3736 showed the lowest antifungal activity (11 mm). This result strongly validates the traditional Ayurvedic use of the oil for conditions like Dadru (ringworm) and other fungal infections.
Conclusion from Results: Marichyadi Taila exhibited moderate antimicrobial activity overall, with the greatest inhibitory efficiency directed against Candida albicans. The formulation demonstrated higher efficacy against Gram-positive bacteria (S. aureus) than Gram-negative bacteria (E. coli). The potency of the oil is significantly lower than the synthetic standard drugs (Gentamycin and Fluconazole), but the effect is consistent across batches and confirms its inherent bioactivity.
Antimicrobial Efficacy of Marichyadi Taila
Discussion
5. Discussion
The study confirms that the traditional Ayurvedic formulation, Marichyadi Taila, possesses significant in-vitro antimicrobial properties, thus scientifically substantiating its classical indication for various skin diseases of infectious etiology. The observed antimicrobial spectrum—inhibition against Gram-negative bacteria (E. coli), Gram-positive bacteria (S. aureus), and fungi (C. albicans)—suggests a broad range of action, which is often characteristic of polyherbal preparations.
.1. Synergistic Action of Constituents
The antimicrobial efficacy of Marichyadi Taila is likely a result of the synergistic combination of its diverse constituents.
-
Antifungal Strength:11 The oil demonstrated its greatest efficiency against C. albicans. This high antifungal potency can be directly attributed to the presence of multiple powerful antimycotic agents: Murchit Katu tail (Mustard oil), Jatamansi (Nardostachys jatamansi), Haridra (Curcuma longa), Devadaru (Cedrus deodara), and Chandana (Santalum album). For instance, curcuminoids from Haridra and sesquiterpenoids from Chandana are well-documented for their fungicidal properties, acting synergistically to disrupt fungal cell membranes. The addition of Gomutra and Gomaya rasa (Cow’s urine and faeces extracts) also contributes known antimicrobial and antifungal components, which are often rich in volatile acids and urea derivatives.
-
Antibacterial Specificity:12 The superior activity against S. aureus (Gram-positive) compared to E. coli (Gram-negative) is a common pattern observed in plant-based antimicrobials. Gram-negative bacteria, like E. coli, possess an outer membrane that acts as an effective barrier, restricting the entry of hydrophobic compounds. In contrast, Gram-positive bacteria, like S. aureus, lack this outer membrane, making their cell walls more susceptible to the lipophilic components of the oil, such as essential oils and fatty acids derived from the base (Mustard oil) and the herbs. Ingredients such as Daru haridra (Berberies aristata) containing Berberine, and the volatile compounds in Maricha (Piper nigrum), are highly effective against Gram-positive cocci.
-
Mineral components: The inclusion of purified mineral components, Haritala (Yellow Arsenic) and Manahshila (Red Arsenic), along with the processed toxin Visha (Aconitum ferox), highlights the traditional reliance on potent, toxic substances for external applications in resistant or chronic skin diseases. While the primary role of Visha and the arsenicals is often described as Kusthaghna (anti-dermatosis), reducing pain, and potentially acting as a catalyst (yogavahi), their specific contribution to the in-vitro antimicrobial zone of inhibition requires further dedicated chemical investigation to separate the effect from the herbal components. Given that the process of Taila Paka (oil preparation) involves complex thermal and chemical reactions, the final form and bioavailability of these mineral components need careful consideration for their topical antimicrobial mechanism.
5.2. Batch-to-Batch Variation
The observed variation in antimicrobial activity across the three batches (e.g., E. coli ZOI from 6 mm to 8 mm; C. albicans ZOI from 11 mm to 15 mm) is a critical finding.
Source of Variation: This variability can stem from several factors inherent in herbal drug manufacturing:
-
Raw Material Quality: Differences in the geographic source, harvest time, processing, and storage of the 18 plant and mineral materials can alter the concentration of key bioactive marker compounds.
-
Processing Parameters: Although standard operating procedures are followed, minor fluctuations in the heating rate, duration of Taila Paka, and the consistency of the Kalka (paste) can influence the final extraction yield of active, thermolabile phytoconstituents into the oil base.
-
Standardization: The need for comprehensive standardization parameters that focus not only on physiochemical identity but also on bioactivity markers is evident to ensure product consistency and reproducible clinical results.
5.3. Comparison with Standard Drugs
Marichyadi Taila's antimicrobial potency was significantly lower than that of Gentamycin and Fluconazole. For example, Gentamycin produced a ZOI of up to 29 mm against S. aureus, whereas the oil peaked at 11 mm. This comparison is expected, as synthetic drugs are purified, high-dose single-molecule entities designed for maximum potency, whereas traditional herbal oils are complex, whole-extract preparations intended for gradual topical effect.
However, the efficacy of the oil should be viewed in the context of its traditional application:
Topical Use: Applied topically, the prolonged contact time and penetration properties of the oil base allow for localized, sustained delivery of the antimicrobial agents, often mitigating the need for the high systemic potency of antibiotics.
Safety Profile: As a natural product, the oil offers a distinct advantage in terms of reduced risk of developing resistance in the same manner as targeted synthetic drugs, and a generally favorable safety profile for topical, non-systemic use.
Conclusion
This in-vitro antimicrobial study confirms that the traditional Ayurvedic formulation, Marichyadi Taila, possesses inherent and broad-spectrum inhibitory activity against tested bacterial (E. coli, S. aureus) and fungal (C. albicans) pathogens. The formulation was consistently more effective against the Gram-positive bacterium Staphylococcus aureus and demonstrated its highest potential against the fungal strain Candida albicans, achieving up to 62.50% of the inhibition efficiency of Fluconazole. The consistent, albeit moderate, efficacy across the tested batches scientifically supports the classical application of Marichyadi Taila in the management of infectious dermatological conditions (Kustha roga).
Future research should focus on isolating the specific bioactive compounds responsible for the observed antimicrobial action, particularly the compounds contributing to the strong antifungal effect. Additionally, minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) studies, alongside detailed in-vivo clinical trials, are necessary to establish the exact dosage and therapeutic range of Marichyadi Taila for clinical use in infectious skin disorders. The present data provides a strong foundational evidence base for the oil's continued utilization in traditional medicine.
References
1. Joshi, Y. M. (2014). Ayurvedic management of skin disorders. Varanasi: Chaukhambha Sanskrit Series Office.
2. Mishra, S. N. (Ed.). (2011). Bhaishajya Ratnavali of Kaviraj Govind Das Sen (The Chaukhamba Ayurvijnan Granthamala 80) [With Sidhiprada Hindi Commentary] (p. 889). Chaukhamba Surbharati Prakashan.
3. Chauhan, A., & Agarwal, M. (2014). A review on the standardization of herbal medicines. International Journal of Bioassays, 3(4), 2375-2380.
4. Kapila, A., & Johar, S. (2025). Preparation and quality control of Marichyadi Taila: An approach to establish Ayurvedic pharmaceutics. International Journal of Advance Research and Innovative Ideas in Education, 11(1), 786–791.
5. Kumar, P., & Singh, P. (2018). A comprehensive review on the pharmacological activities of Piper nigrum (Black Pepper). International Journal of Current Microbiology and Applied Sciences, 7(1), 2597-2606.
6. Mukherjee, P. K. (2002). Quality control of herbal drugs: An approach to evaluation of botanicals. Business Horizons.
7. Patwardhan, B., Warude, D., Pushpangadan, P., & Bhatt, N. (2005). Ayurveda and traditional Indian medicine: A reconsideration of concepts, therapeutic approaches, and policy concerns. Journal of Alternative and Complementary Medicine, 11(5), 771-776.
8. Bose S, Singh DV, Adhya TK, Acharya N. Escherichia coli, but not Staphylococcus aureus, functions as a chelating agent that exhibits antifungal activity against the pathogenic yeast Candida albicans. Journal of Fungi. 2023 Feb 22;9(3):286.
9. Kumar, S., & Sharma, R. (2023). Standardization and quality control analysis of Marichadi Tailam: An Ayurvedic formulation. Asian Journal of Research in Chemistry, 16(2), 78–83.
10. Rose SB, Miller RE. Studies with the agar cup-plate method: I. A standardized agar cup-plate technique. Journal of bacteriology. 1939 Nov;38(5):525-37.
11. Bravo-Chaucanés CP, Vargas-Casanova Y, Chitiva-Chitiva LC, Ceballos-Garzon A, Modesti-Costa G, Parra-Giraldo CM. Evaluation of anti-Candida potential of Piper nigrum extract in inhibiting growth, yeast-hyphal transition, virulent enzymes, and biofilm formation. Journal of Fungi. 2022 Jul 27;8(8):784.
12. Patil T, Wele A. Significance of pharmacokinetics of Marich [Piper nigrum (L)] as a possible marker and bioavailability enhancer of Ayurvedic Formulations. INDIAN DRUGS. 2007 May;44:5.

